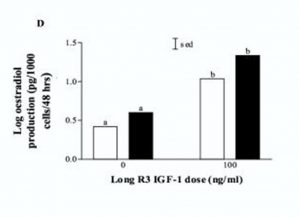
Effects of IGF-1 LR3 on the estradiol production in porcine granulosa cells (‘b’ indicates a significant difference). From Brankin V, Mitchell MR, Webb B, Hunter MG. Paracrine effects of oocyte secreted factors and stem cell factor on porcine granulosa and theca cells in vitro. Reproductive biology and endocrinology : RB&E. 2003;1:55, reproduced under the terms of the Creative Commons Attribution License
Insulin-like growth factor-1 (IGF-1) is a protein with a role in growth hormone- (GH) induced signaling, development and other functions
1. For example, the IGF-1 levels of some mammalian species change in response to feeding and changes in the amounts of food-generated energy
2. IGF-1 is regarded as a peptide hormone, an endocrine factor and a paracrine factor, depending on the part of the body in which it is active
3. It is available as a laboratory-grade compound known as IGF-1 LR3. This synthetic analog of IGF-1 has an arginine residue in place of the normal glutamic acid in the third position along its sequence. IGF-1 LR3 also has an elongated amino-terminus, to give a protein of 83 amino acids as opposed to the 70 found in the natural or endogenous molecule. These variations impair the ability of IGF-binding proteins (IGFBP)
in vivo or
in vitro1. IGFBPs are involved in the regulation of IGF-1 and its activity at the IGF receptor. Therefore, IGF-1 LR3 may be more potent when administered in comparison to normal IGF-1
1. On the other hand, it may also have greater clearance from the blood due to the lack of binding. The affinity of IGF-1 for the IGF receptor is poor compared to that of IGFBPs for IGF-1
1. Therefore, binding proteins can exert considerable negative regulation on IGF receptor activation. (Conversely, some IGFBP subtypes promote IGF-1 activity, depending on a number of factors including their phosphorylation status
1.) Modified IGF can have a 100-fold (or more) reduction in affinity for IGFBPs compared to the original molecule
1. IGF-1 LR3 may have a potency that is approximately two-fold greater than that of IGF-1, as evidenced by a study using rats
4. IGF-1 has a molecular weight of about 7kDa; IGF-1 LR3 may be a little larger than this
4.
Treatment with IGF-1 LR3 is associated with significant increases in sodium ion flux across the gut epithelium of sheep
2. This indicates a role for IGF-1 in the essential nerve cell activity, motility and pH regulation in the digestive tract. IGF-1 LR3 has also been used to assess the role of IGF-1 in follicle development in mammals. Both IGF-1 LR3 and recombinant human IGF-1 were associated with dose-dependent increases in the size and estradiol release of cultured bovine follicles
5. However, higher doses of IGF-1 resulted in reduced oocyte numbers, indicating that follicular development depends on the tight regulation of IGF-1 activity
5. This may be supported by the detection of IGFBP2 and of IGFBP3 mRNA in the cultured follicles
5. IGF-1 LR3 may also be used to assess the overexpression of IGF receptors often observed in tumor cells
6. It is associated with cyclin D1 activity, a marker of cell cycle activity, mediated by the IGF receptor in these cells
6. The function of this is to enhance the proliferation, survival and invasive migration of tumors
6. IGF-1 LR3 may also be applied to studies assessing novel antagonists of this receptor intended to prevent this. Another function of IGF-1 LR3 is to assess the expression patterns of IGFBPs in various tissues. For example, a recent study found that these are not regulated by the presence of IGF in murine skeletal muscle
7.
References:
- Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. The Journal of endocrinology. 2002;175(1):19-31.
- Shen Z, Martens H, Schweigel-Rontgen M. Na+ transport across rumen epithelium of hay-fed sheep is acutely stimulated by the peptide IGF-1 in vitro. Experimental physiology. 2012;97(4):497-505.
- Brankin V, Mitchell MR, Webb B, Hunter MG. Paracrine effects of oocyte secreted factors and stem cell factor on porcine granulosa and theca cells in vitro. Reproductive biology and endocrinology : RB&E. 2003;1:55.
- Tomas FM, Lemmey AB, Read LC, Ballard FJ. Superior potency of infused IGF-I analogues which bind poorly to IGF-binding proteins is maintained when administered by injection. The Journal of endocrinology. 1996;150(1):77-84.
- Thomas FH, Campbell BK, Armstrong DG, Telfer EE. Effects of IGF-I bioavailability on bovine preantral follicular development in vitro. Reproduction (Cambridge, England). 2007;133(6):1121-1128.
- Haluska P, Carboni JM, Loegering DA, et al. In vitro and in vivo antitumor effects of the dual insulin-like growth factor-I/insulin receptor inhibitor, BMS-554417. Cancer research. 2006;66(1):362-371.
- Oliver WT, Rosenberger J, Lopez R, Gomez A, Cummings KK, Fiorotto ML. The local expression and abundance of insulin-like growth factor (IGF) binding proteins in skeletal muscle are regulated by age and gender but not local IGF-I in vivo. Endocrinology. 2005;146(12):5455-5462.



