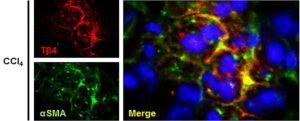
- Cells containing thymosin beta-4 (Tβ4) co-localize to alpha-smooth muscle actin (alphaSMA), indicating that the protein is active in the presence of fibrosis (induced by experimental chronic liver damage generated by treatment with CCI4). From: Kim J, Wang S, Hyun J, et al. Hepatic Stellate Cells Express Thymosin Beta 4 in Chronically Damaged Liver. PloS one. 2015;10(3):e0122758, reproduced under the terms of the Creative Commons Attribution License
Thymosin beta-4 is a protein originally discovered as an isolate from the mammalian thymus gland
1. It is one of a family of thymosins, which are low-weight acidic molecules that can act as cytopoietics. This means that they can control the movement and/or differentiation of cells by neutralizing the ability of individual actin proteins (or monomers) to polymerize into filaments
2. On the other hand, thymosins also aggregate the actin monomers, thus allowing or preventing the formation of subsequent filaments
2. At a certain scale, this may promote or prevent the differentiation of a cell such as a pluripotent stem cell into another, such as an osteocyte or a neural cell. Therefore, molecules such as thymosins (of which thymosin beta-4 is the most common in mammals
3) may play a significant role in postnatal development or the regeneration of some tissues. The influence of thymosin beta-4 over actin may also control cellular migration and angiogenesis
4. It follows, therefore, that the protein also has potential as an agent of wound repair. Thymosin beta-4 is available as a synthetic, laboratory-grade peptide known as TB500
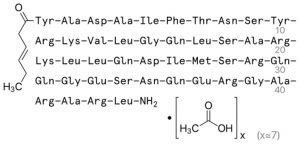
i. Its molecular weight is just over 4.9kDa, and it is supplied as a white solid
i.
TB500 also has anti-inflammatory properties. It has demonstrated the ability to attenuate the release of NO and prostaglandin E4 in cellular models of reactive oxygen species (ROS) exposure
5. However, it may also upregulate pro-inflammatory cytokines such as TNFalpha and several interleukins (e.g. IL-6 and -8) in periodontal cells
5. As these molecules are also osteoclastogenic
5, it implies an additional role for TB500 in the regulation of bone formation. The peptide also inhibited the activation of NFkappaB in murine macrophages in this study
5. TB500 also concerns the release of acSDKP, an anti-inflammatory peptide fragment. This fragment is in fact a breakdown product of TB500, but this metabolism is regulated by a number of interesting factors
6. The co-incubation of TB500 with homogenated rodent kidney tissue resulted in a significant increase in the release of acSDKP
7. This is controlled by a complex regulatory mechanism which involves peptidases that only cleave molecules of specific fragments, necessitating the hydrolysis of TB500 by meprin-alpha before acSDKP may be cleaved from it
7. TB500 may also be of interest to researchers studying the fibrosis of various organs
8. One group has recently published results that indicate significant decreases of inflammation in a mouse model of pulmonary fibrosis
9. acSDKP has been shown to decrease renal fibrosis in mice, in that treatment with the fragment resulted in the reduced deposition of fibronectin and collagen (i.e. major components of scar tissue) and the reduced migration of macrophages and myofibroblasts to a site of damage
6. The up-regulation of thymosin beta-4 is also associated with the activation of hepatic stellate cells, which appear to co-localize to alpha-smooth muscle actin (a marker of chronic liver damage as observed in mouse models of the same)
10.
In general, TB500 is a peptide involved in complex regulatory and developmental processes
in vivo. It appears to be a viable component of the control of tissue regeneration, cell differentiation and inflammation. Therefore, B500 may be applied to models of disorders such as abnormal fibrosis, osteolytic inflammation (e.g. rheumatoid arthritis) and various states of postnatal development.
References:
- Goldstein AL, Slater FD, White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proceedings of the National Academy of Sciences of the United States of America. 1966;56(3):1010-1017.
- Huff T, Muller CS, Otto AM, Netzker R, Hannappel E. beta-Thymosins, small acidic peptides with multiple functions. The international journal of biochemistry & cell biology. 2001;33(3):205-220.
- Cha HJ, Philp D, Lee SH, Moon HS, Kleinman HK, Nakamura T. Over-expression of thymosin beta 4 promotes abnormal tooth development and stimulation of hair growth. The International journal of developmental biology. 2010;54(1):135-140.
- Philp D, Goldstein AL, Kleinman HK. Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development. Mechanisms of ageing and development. 2004;125(2):113-115.
- Lee S-I, Yi J-K, Bae W-J, Lee S, Cha H-J, Kim E-C. Thymosin Beta-4 Suppresses Osteoclastic Differentiation and Inflammatory Responses in Human Periodontal Ligament Cells. PloS one. 2016;11(1):e0146708.
- Zuo Y, Chun B, Potthoff SA, et al. Thymosin beta4 and its degradation product, Ac-SDKP, are novel reparative factors in renal fibrosis. Kidney international. 2013;84(6):1166-1175.
- Kumar N, Nakagawa P, Janic B, et al. The anti-inflammatory peptide Ac-SDKP is released from thymosin beta4 by renal meprin alpha and prolyl oligopeptidase. American journal of physiology. Renal physiology. 2016:ajprenal.00562.02015.
- Peng H, Xu J, Yang XP, et al. Thymosin-beta4 prevents cardiac rupture and improves cardiac function in mice with myocardial infarction. American journal of physiology. Heart and circulatory physiology. 2014;307(5):H741-751.
- Conte E, Genovese T, Gili E, et al. Protective effects of thymosin beta4 in a mouse model of lung fibrosis. Annals of the New York Academy of Sciences. 2012;1269:69-73.
- Kim J, Wang S, Hyun J, et al. Hepatic Stellate Cells Express Thymosin Beta 4 in Chronically Damaged Liver. PloS one. 2015;10(3):e0122758.
- TB500 Product Page. Blue Sky Peptide. 2016