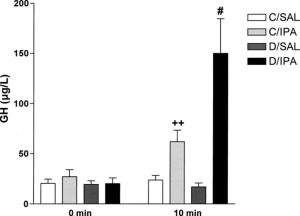
The effect of ipamorelin treatment on diabetic (D/IPA) and healthy (C/IPA) mice compared to saline-treated controls. From: Johansen PB, Segev Y, Landau D, Phillip M, Flyvbjerg A. Growth hormone (GH) hypersecretion and GH receptor resistance in streptozotocin diabetic mice in response to a GH secretagogue. Experimental diabesity research. 2003;4(2):73-81, reproduced under the terms of the Creative Commons Attribution License
Ipamorelin is a hexapeptide (or six-amino-acid) that was developed to mimic ghrelin and its
in vivo properties. Until other similar molecules, such as GHRP-1, -2 and -6, ipamorelin contains synthetic amino acids
1. The main function of ipamorelin is to increase the production of growth hormone (GH) from cells that express the GHS-R receptor (also known as the ghrelin receptor). It may also increase IGF-1 levels in healthy animals
2. However, other similar molecules, such as those listed above, may be associated with significant increases in ACTH and cortisol, whereas ipamorelin is not
1. Nevertheless, ipamorelin may affect growth and development in a manner similar to GH and other ghrelin mimetics (i.e. the GHRPs). Female rats were treated with comparable doses of GH, GHRP and ipamorelin for eight-weeks. This resulted in similar and significant increases in body weight for all three treatments
3. Therefore, ipamorelin may be used as a comparative reagent in the assessment of GHS-R activation and GH release in novel or less-studied species
4. It may also be applied to studies investigating the effects of GH release on subsequent GH expression in these species. For example, ipamorelin was recently incorporated into a study indicating that GH release does not depend on or is influenced by GH mRNA transcription in the black sea bream
4. (The expression of GH and GHS-R activation act synergistically, or as a positive feedback loop, in some mammalian species
4. This can also be confirmed through the use of ipamorelin.) Therefore, ipamorelin may be active at many cell types that release GH
in vivo. These include, but are not limited to, pituitary, cardiac and liver cells.
GH is also associated with a role in normal bone growth and structure. Therefore, ipamorelin may also have a role in bone formation. A study compared bone mass (measured as bone mineral density) in rats treated with 0.5 mg/kg ipamorelin, 0.5 mg/kg GHRP-6, 3.5 mg/kg GH or a placebo every day for eight weeks. Ipamorelin was associated with significant increases in post mortem bone mineral content scans, although to a lesser extent than GH or GHRP-6, compared to placebo
3. However, the total weights of vertebrae from ipamorelin-treated rats were significantly greater than those from rats in the placebo group, and were comparable to those taken from GH- and ghrp-6-treated rats
3. Ipamorelin also appears to have beneficial effect in animal models of diabetes, as does ghrelin
5. The peptide has demonstrated the ability to increase insulin production in the pancreatic tissue of diabetic and non-diabetic rats
6. Unlike ghrelin, however, ipamorelin appears to elicit these effects via adrenergic receptors and through calcium channel activation
6. Treatment with ipamorelin also results in significant increases in GH concentrations in diabetic mice when compared to non-diabetic mice
2. This indicates GH resistance, an indicator of type 1 diabetes. Normal mice treated with ipamorelin exhibited significant increases in hepatic IGF-1 compared to identical controls, whereas diabetic, treated mice showed significantly reduced levels of this protein compared to their healthy counterparts
2. These results demonstrate how ipamorelin can be used to highlight the abnormal hepatic response to GH in this model of diabetes.
References:
- Raun K, Hansen BS, Johansen NL, et al. Ipamorelin, the first selective growth hormone secretagogue. European journal of endocrinology / European Federation of Endocrine Societies. 1998;139(5):552-561.
- Johansen PB, Segev Y, Landau D, Phillip M, Flyvbjerg A. Growth hormone (GH) hypersecretion and GH receptor resistance in streptozotocin diabetic mice in response to a GH secretagogue. Experimental diabesity research. 2003;4(2):73-81.
- Svensson J, Lall S, Dickson SL, et al. The GH secretagogues ipamorelin and GH-releasing peptide-6 increase bone mineral content in adult female rats. The Journal of endocrinology. 2000;165(3):569-577.
- Chan CB, Fung CK, Fung W, Tse MC, Cheng CH. Stimulation of growth hormone secretion from seabream pituitary cells in primary culture by growth hormone secretagogues is independent of growth hormone transcription. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP. 2004;139(1-3):77-85.
- Adeghate E, Ponery AS. Ghrelin stimulates insulin secretion from the pancreas of normal and diabetic rats. J Neuroendocrinol. 2002;14(7):555-560.
- Adeghate E, Ponery AS. Mechanism of ipamorelin-evoked insulin release from the pancreas of normal and diabetic rats. Neuro Endocrinol Lett. 2004;25(6):403-406.