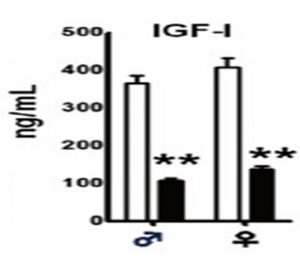
IGF1 levels in mice with GRF signaling deficiency (black bars) compared to normal controls (white bars). Asterisks indicate significant differences. From Sun LY, Spong A, Swindell WR, et al., Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. eLife. 2013;2:e01098, reproduced under the terms of the Creative Commons Attribution License
- Qin YJ, Chan SO, Chong KK, et al. Antagonist of GH-releasing hormone receptors alleviates experimental ocular inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(51):18303-18308.
- Gaudreau P, Boulanger L, Abribat T. Affinity of human growth hormone-releasing factor (1-29)NH2 analogues for GRF binding sites in rat adenopituitary. Journal of medicinal chemistry. 1992;35(10):1864-1869.
- Jette L, Leger R, Thibaudeau K, et al. Human growth hormone-releasing factor (hGRF)1-29-albumin bioconjugates activate the GRF receptor on the anterior pituitary in rats: identification of CJC-1295 as a long-lasting GRF analog. Endocrinology. 2005;146(7):3052-3058.
- Talhouk RS, Saade NE, Mouneimne G, Masaad CA, Safieh-Garabedian B. Growth hormone releasing hormone reverses endotoxin-induced localized inflammatory hyperalgesia without reducing the upregulated cytokines, nerve growth factor and gelatinase activity. Progress in neuro-psychopharmacology & biological psychiatry. 2004;28(4):625-631.
- Khorram O, Yeung M, Vu L, Yen SS. Effects of [norleucine27]growth hormone-releasing hormone (GHRH) (1-29)-NH2 administration on the immune system of aging men and women. The Journal of clinical endocrinology and metabolism. 1997;82(11):3590-3596.
- Estevez MD, Alfonso A, Vieytes MR, Louzao MC, Botana LM. Study of the activation mechanism of human GRF(1-29)NH2 on rat mast cell histamine release. Inflammation research : official journal of the European Histamine Research Society ... [et al.]. 1995;44(2):87-91.
- Ziegler CG, Ullrich M, Schally AV, et al. Anti-tumor effects of peptide analogs targeting neuropeptide hormone receptors on mouse pheochromocytoma cells. Molecular and cellular endocrinology. 2013;371(0):189-194.
- Munoz-Moreno L, Arenas MI, Carmena MJ, Schally AV, Prieto JC, Bajo AM. Growth hormone-releasing hormone antagonists abolish the transactivation of human epidermal growth factor receptors in advanced prostate cancer models. Investigational new drugs. 2014;32(5):871-882.
- Perez R, Schally AV, Vidaurre I, Rincon R, Block NL, Rick FG. Antagonists of growth hormone-releasing hormone suppress in vivo tumor growth and gene expression in triple negative breast cancers. Oncotarget. 2012;3(9):988-997.
- Stangelberger A, Schally AV, Rick FG, et al. Inhibitory effects of antagonists of growth hormone releasing hormone on experimental prostate cancers are associated with upregulation of wild-type p53 and decrease in p21 and mutant p53 proteins. The Prostate. 2012;72(5):555-565.
- Stepien T, Sacewicz M, Lawnicka H, et al. Stimulatory effect of growth hormone-releasing hormone (GHRH(1-29)NH2) on the proliferation, VEGF and chromogranin A secretion by human neuroendocrine tumor cell line NCI-H727 in vitro. Neuropeptides. 2009;43(5):397-400.
- Wang T, Hai J, Chen X, et al. Inhibition of GHRH aggravated acetaminophen-induced acute mice liver injury through GH/IGF-I axis. Endocrine journal. 2012;59(7):579-587.