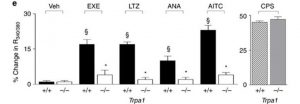
The response to exemestane in normal neurons (+/+) compared to that of TRPA1-deficient (-/-) cells. CPS=capsaicin, a control treatment demonstrating the presence of other similar channels. From: Fusi C, Materazzi S, Benemei S, et al. Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nature communications. 2014;5:5736, reproduced under the terms of a Creative Commons Attribution 4.0 International License
Exemestane is a steroidal aromatase inhibitor (AI)
1. Unlike many other AIs, its bond to the aromatase protein is irreversible
2. Aromatase is associated with the final step in estrogen synthesis
2. Therefore, exemestane may be used to impair the production of estrogen in the study and treatment of cancers associated with the abnormal function of this hormone and/or its receptors
2. Exemestane may also demonstrate the efficiency of combined therapies (as opposed to monotherapies) proposed to address these diseases
3. It is highly hydrophilic, and may react with the free thiol groups on the cysteine residues of many proteins
2.
Exemestane and Estrogen-Specific Cancers
A combination of cisplatin and exemestane elicited the greatest response to treatment in a rat model of ovarian cancer
3. The administration of this combination (in female Wistar rats with experimentally-induced ER-positive tumors) also resulted in significant reductions in a marker of angiogenesis, although a combination of the GnRH agonist triporelin and cisplatin was superior in this respect
3.
Exemestane and Other Cancers
This peptide may also be effective in the treatment of other cancers with which aromatase expression is thought to be associated. This includes malignant pleural mesothelioma (MPM). A study on the effect of exemestane on cultured MPM cells found that the peptide elicited dose-dependent reductions in the proliferation and metabolic activity of these cells, which were significant at higher (35μM or more) doses
4. This was found to be associated with a modulation of cAMP, and thus CD44, that resulted from treatment with exemestane
4.
Exemestane and Pain Biology
Many researchers have observed that treatment with exemestane and other similar AIs results in the side effect of pain. This is thought to be associated with certain TRPA channels in animals. The TRPA family of proteins is also associated with the neurobiological response to irritants such as wasabi (or its active ingredient, AITC)
5. A study investigated the response of the TRPA1 channel, expressed on cell lines, to exemestane and other peptides in its category. Treatment with exemestane elicited the highest calcium response from the channels compared to that with anastrozole and letrozole, and resulted in an EC
50 of 58μM, compared to 134 for anastrozole and 69 for letrozole
1. All responses were significantly different compared to an identical placebo treatment
1. These effects were abolished by a selective antagonist for TRPA1
1. These effects were confirmed using cultured mouse and rat dorsal root ganglion neurons on which TRPA1s are present (i.e. 'pain' neurons). Exemestane and other AIs elicited similar calcium responses, with an EC
50 of 82μM for exemestane
1. The responses in normal mouse neurons were then compared to those from TRPA1-deficient mice. This resulted in significantly greater responses in the normal cells to exemestane, compared to that in the 'deficient' cells
1.
Exemestane and Antioxidant Pathways
Exemestane has been found to have remarkable homology with inducer proteins associated with the genes regulated by the Keap1/Nrf2/ARE signaling pathway
2. In other words, the peptide may have potential as an antioxidant. Some researchers have demonstrated the ability of exemestane to activate the enzymes NQO1 and HO1, which may be associated with reduced damage in cultured cells following exposure to UV and hypoxia
2. It can also protect cells against oxidative damage caused by 4-hydroxynonenal - which is also an activator of the TRPA1 channel
2,6. Therefore, exemestane may protect against cancer development and oxidative stress, albeit with the drawback of hyperalgesia and/or various types of pain
in vivo.
References:
- Fusi C, Materazzi S, Benemei S, et al. Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nature communications. 2014;5:5736.
- Liu H, Talalay P. Relevance of anti-inflammatory and antioxidant activities of exemestane and synergism with sulforaphane for disease prevention. Proc Natl Acad Sci U S A. 2013;110(47):19065-19070.
- Tkalia IG, Vorobyova LI, Grabovoy AN, Svintsitsky VS, Tarasova TO. The antitumor efficacy of cisplatin in combination with triptorelin and exemestane therapy for an ovarian cancer ascites model in Wistar rats. Experimental oncology. 2015;37(1):30-35.
- Nuvoli B, Germoni S, Morosetti C, et al. Exemestane blocks mesothelioma growth through downregulation of cAMP, pCREB and CD44 implicating new treatment option in patients affected by this disease. Molecular cancer. 2014;13:69.
- Kurganov E, Zhou Y, Saito S, Tominaga M. Heat and AITC activate green anole TRPA1 in a membrane-delimited manner. Pflugers Archiv : European journal of physiology. 2014;466(10):1873-1884.
- Trevisani M, Siemens J, Materazzi S, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104(33):13519-13524.



